Parkinson’s disease - Biomarkers
Parkinson’s Disease (PD) is the second most common neurodegenerative disorder, characterized by the loss of dopaminergic neurons and the accumulation of aggregated α-synuclein, mainly in the substantia nigra pars compacta (SNpc) of the midbrain.
The hallmark motor symptoms include tremor, bradykinesia, postural instability, and rigidity, often accompanied by non-motor issues such as dementia, depression, and psychosis. Protein biomarkers in Parkinson’s disease (PD) are critical for understanding disease mechanisms, early diagnosis, and developing therapeutic strategies.
Several key biomarkers have been identified and investigated in PD research:
Hypothetical model of α-syn toxicity and spread of pathology in PD (Image taken from: Irwin DJ, …, Trojanowski JQ. Nat Rev Neurosci. 2013) 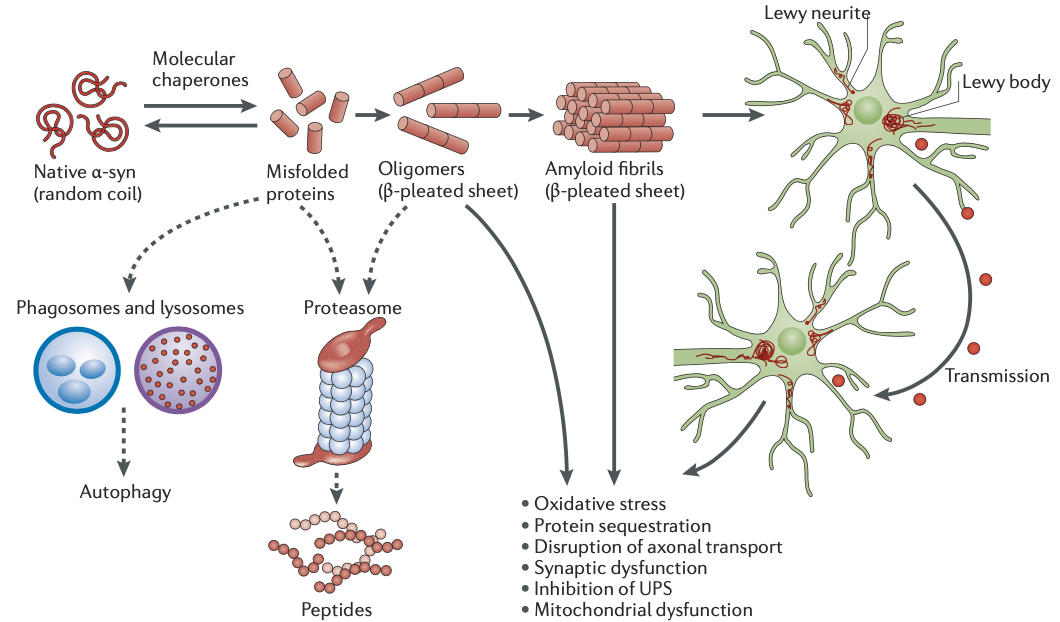
Alpha-synuclein (α-synuclein): The most prominent protein associated with PD. Aggregation of α-synuclein into Lewy bodies is a hallmark of the disease. Detecting abnormal forms of α-synuclein in cerebrospinal fluid (CSF), blood, and even saliva is a major focus of biomarker research.
DJ-1 (PARK7): DJ-1 is involved in antioxidative stress mechanisms. Lower levels of DJ-1 have been observed in the CSF of PD patients, making it a potential biomarker for early diagnosis.
Tau Protein: Although primarily linked to Alzheimer’s disease, tau has been studied in PD, particularly in cases where Parkinsonism overlaps with dementia. Altered tau levels in CSF may help differentiate PD from other neurodegenerative diseases.
Neurofilament Light Chain (NfL): This structural protein is released into the CSF and blood during neurodegeneration. Elevated NfL levels are indicative of neuronal damage and may serve as a biomarker for disease progression in PD.
Urate: Low levels of urate in the blood have been associated with an increased risk of developing PD. It has antioxidant properties, and some studies suggest it could be protective against neurodegeneration.
LRRK2 (Leucine-rich repeat kinase 2): Mutations in the LRRK2 gene are a common genetic cause of PD, and increased kinase activity has been observed in PD patients. LRRK2 is being studied both as a genetic biomarker and therapeutic target.
Glial Fibrillary Acidic Protein (GFAP): GFAP is a marker of astroglial activation. Elevated levels in biofluids suggest gliosis, which is associated with neurodegenerative diseases, including PD.
Identifying and validating these and other protein biomarkers is ongoing, with many studies focusing on combinations of markers for more accurate diagnosis and monitoring disease progression.
Table: Proposed pathogenic mechanisms in Parkinson’s disease (Modified table from Morris HR,…, Williams-Gray CH. Lancet. 2024)
| Proposed Pathogenesis | Proposed Mechanism | Genetic Evidence | Biomarkers |
|---|---|---|---|
| Increase in SNCA expression | Increase in α-synuclein protein leads to increased aggregation and cell death | Increase in SNCA gene dose (duplications or triplications) causes Parkinson’s disease; SNCA common variants probably lead to increased expression | α-Synuclein and phospho-synuclein measurement in blood and CSF |
| Increase in α-synuclein aggregation | Formation of oligomers and fibrils leads to cellular toxicity | Coding mutations in SNCA lead to increase in α-synuclein aggregation | rt-QUIC assays for aggregation based on CSF, skin biopsies, olfactory mucosal biopsies, and saliva |
| Mitochondrial dysfunction | Reduced complex 1 activity, abnormal calcium homeostasis, increased reactive oxygen species, reduced mitochondrial ATP production | Multiple Parkinson’s disease gene mutations lead to changes in mitochondrial function, including PRKN, PINK1, and LRRK2 | Magnetic resonance spectroscopy analysis of Pi to ATP ratios, measurement of ATP, and mitochondrial function in skin fibroblasts |
| Altered endosomal-lysosomal trafficking | Activation of LRRK2 and VPS35 leads to decreased lysosomal function and altered response to membrane damage | Rare pathogenic variants in LRRK2 (e.g., Gly2019Ser) and VPS35 lead to increased Rab phosphorylation | Measurement of Rab protein phosphorylation in cells from peripheral blood; measurement of urinary BMP phospholipids |
| Lysosomal dysfunction | Impaired α-synuclein degradation leads to increased cellular α-synuclein | GBA1 mutations are associated with Parkinson’s disease, and rare variants in other genes might be relevant | Measurement of GCase protein and enzyme activity; measurement of GSLs in blood and CSF; measurement of urinary BMP phospholipids |
| Immune activation and neuroinflammation | Aggregated α-synuclein, mitochondrial antigens, and gut bacterial endotoxins promote immune responses and neuroinflammation | Association between HLA variants and Parkinson’s disease; LRRK2, PRKN, and PINK1 are involved in inflammatory pathways | Measurement of C-reactive protein, interleukins, and PET imaging of activated microglia |
| Cell-to-cell spread | Toxic forms of α-synuclein spread between contiguous cells or via extracellular vesicles | NA | rt-QUIC assays for aggregation based on CSF, skin biopsies, olfactory mucosal biopsies, and saliva |
A tremor is a shaking movement, most commonly seen in the hands and arms. Bradykinesia is the slowing down of movement and can include trouble starting movements or even a complete lack of movement.
